OPTIMA IPL

Treat the root cause: chronic inflammation
Optima IPL is a drug free, drop free light-based treatment, which targets the root causes – chronic inflammation.
Optima IPL treats periocular inflammatory conditions such as Rosacea and telangiectasia, which may lead to ocular surface conditions such as Dry Eye6,7. 80% of rosacea patients suffer from Meibomian Gland Dysfunction.
STOP THE VICIOUS CYCLE
OF INFLAMMATION
Reduces inflammatory mediators
Decreases the level of pro inflammatory mediators, progressing the inflammation3, 4
Alleviates Abnormal Blood vessels
Destroys the abnormal blood vessels that are perpetuating the inflammation1,2
Decreases Demodex
Decreases Demodex mites which are stimulating infection leading to inflammation5
ENERGY TO
EYE CARE
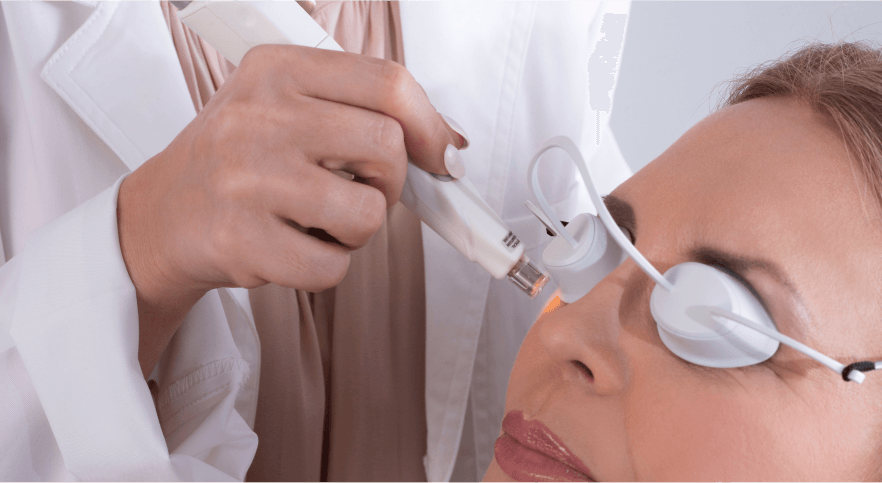
Uniquely designed for the periocular region
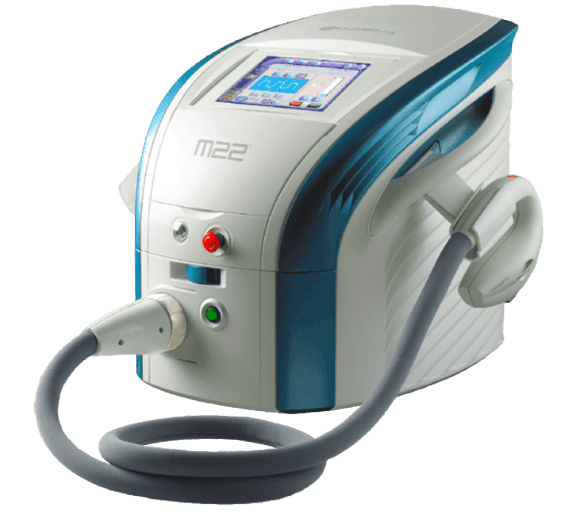
The Optima IPL is the next-generation IPL technology developed by Lumenis.
With every pulse of light, this first-of-its-kind, energy-based therapy works to
control the inflammatory process and to stop the vicious cycle of inflammation
Reducing the need for medications and providing long-lasting results.
LUMENIS PATENTED
TECHNOLOGIES
Enables to treat with up to 20 J/cm2, gently and safely for maximum effectiveness
Optima IPL with OPT™
SAFETY
No spikes in energy The energy you choose is the energy you get
EFFICACY
Consistent level of energy in every pulse, throughout the pulse

Patented water cool tips: perfect fit for eye-care SapphireCool™ chiller tip allows to:
1. Reach higher levels of energy and achieve optimal results
2. Provide safe and comfortable treatment for the patient
3. Small tip for better access to eyelid area
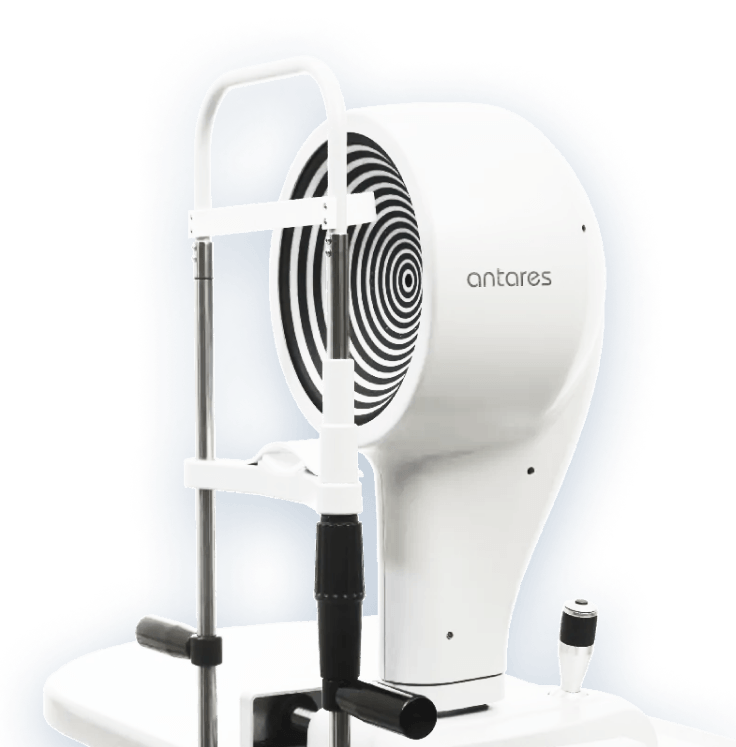
ANTARES DRY EYE
DISEASE DIAGNOSTICS
Advanced, automated DED diagnostic and corneal topographer
Quality
Accurate, reliable, reproducible and low intra-rater variability
Speed
Fast examination process and results
Comfortable
Offers patient comfort as well as physician ease of use
Applications
Dry eye report, Meibography, Tear Breakup Time, Tear Meniscus, Topographer
WHAT ARE THE
EXPERTS SAYING?
Must have in the ophthalmic toolbox
Tal Raviv, MD, Eye Center of New York
“The Optima IPL has not just been another addition to our Toolbox, it has become one of the primary workhorses in our practice to achieve outstanding patient outcomes”
Neil Desai, MD
“Optima IPL has become such a powerful tool in my tool box because it is uniquely and for the first time gives me a method by which I can treat the source of the problem”
OPTIMA IPL
SPECIFICATIONS
ExpertFilters
515 nm; 560 nm; 590 nm; 615 nm; 640 nm; 695 nm; 755nm
Lightguides
15 x 35 mm; 8 x 15 mm
Fluence
Up to 35 J/cm2
Cooling
Continuous contact cooling
Risk and warning (non-inclusive list): Treatment with M22 Optima IPL is contraindicated for patients with the following conditions in the treatment area: active infections, dysplastic nevi, tattoos, significant concurrent skin inflammation (excluding rosacea or acne), active cold sores, open lacerations, abrasions, and prolonged sun-exposure. Caution is advised for patients with a compromised immune system, coagulation disorders, photosensitivity, hormonal disorders, or a history of Herpes simplex near the treatment area. Patients eyes must be occluded with eye shields during the treatment.
1. Kassir et al. (2011) J Cosmet Laser Ther 13(5):216-22; 2. Papageorgiou et al. (2008) Br J Dermatol 159(3):628-32; 3. Liu et
al. (2017) Am J Ophthalmol 183:81-90.; 4. Yin et al. (2018) Curr Eye Res 43(3):308-13; 5. Prieto et al. (2002) Lasers Surg Med 30(2):82-5; 6. De Paiva et al. (2006) Am J Ophthalmol 141(3):438-45; 7. Solomon et al. (2004) Ocul Surf 2(1):34-44;
*Antares is manufactured for Lumenis inc. by Costruzione Strumeni Oftalmici
PB-00001770 Rev A











